
Simplify & Harmonize Pharmacovigilance Aggregate Safety Reports
WEBINAR Simplify & Harmonize Pharmacovigilance Aggregate Safety Reports Thursday, 15
Too often, pharmacovigilance and risk management experts spend their time and expertise grappling with technical limitations just to stay on track. Orbit helps teams like yours bridge the gaps in your current safety systems with our cross-functional process management platform.
Orbit is a strategic platform for patient safety, helping small, medium, and large biopharma companies scale their safety operations across global markets.
Track global RMP commitments and support local Additional Risk Minimization Measure implementation.
Manage PVA and SDEA content and commitments to gain oversight across global partnerships.
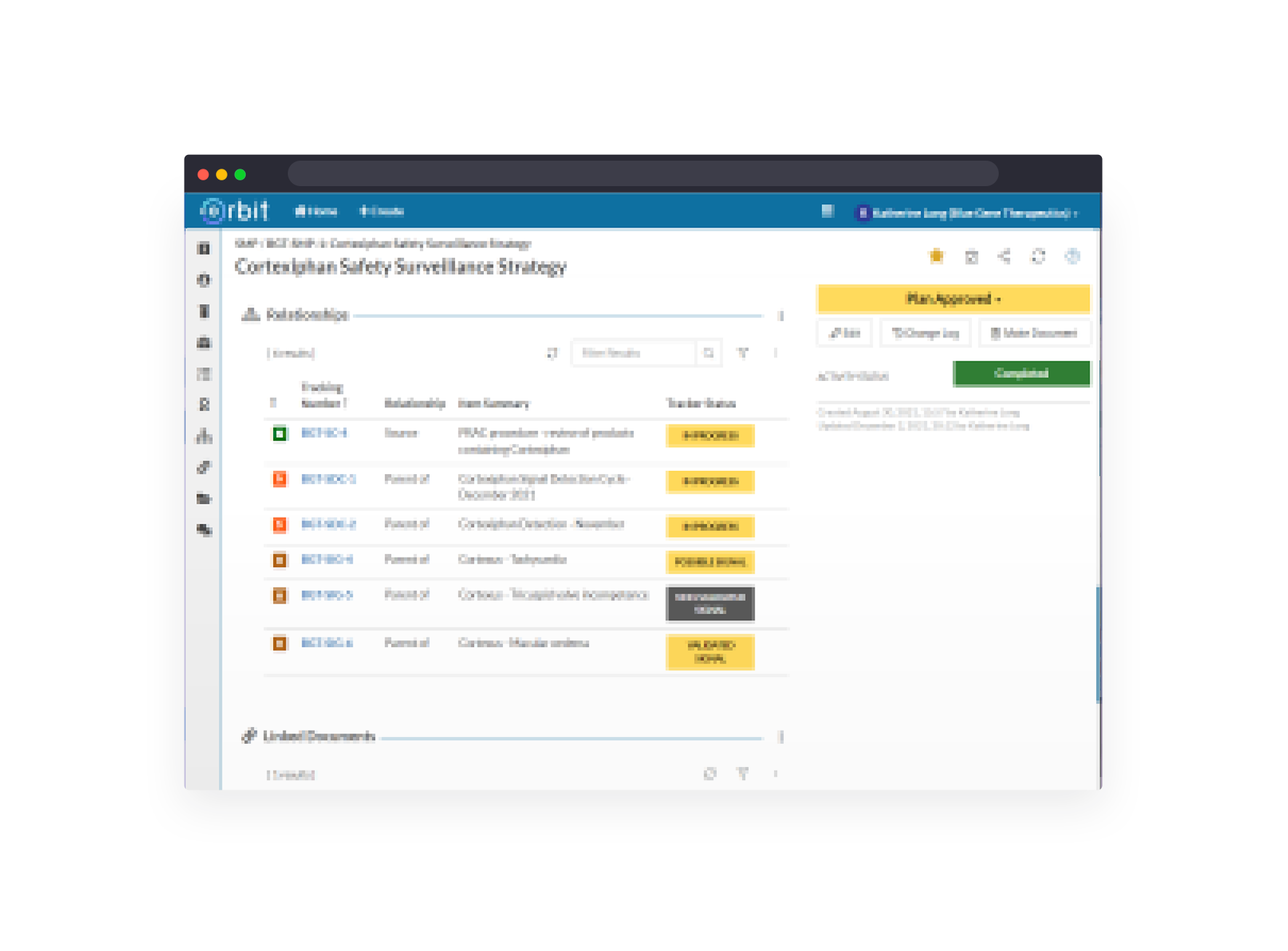
Module IX signal management from strategy through detection activities, signal assessment and tracking.
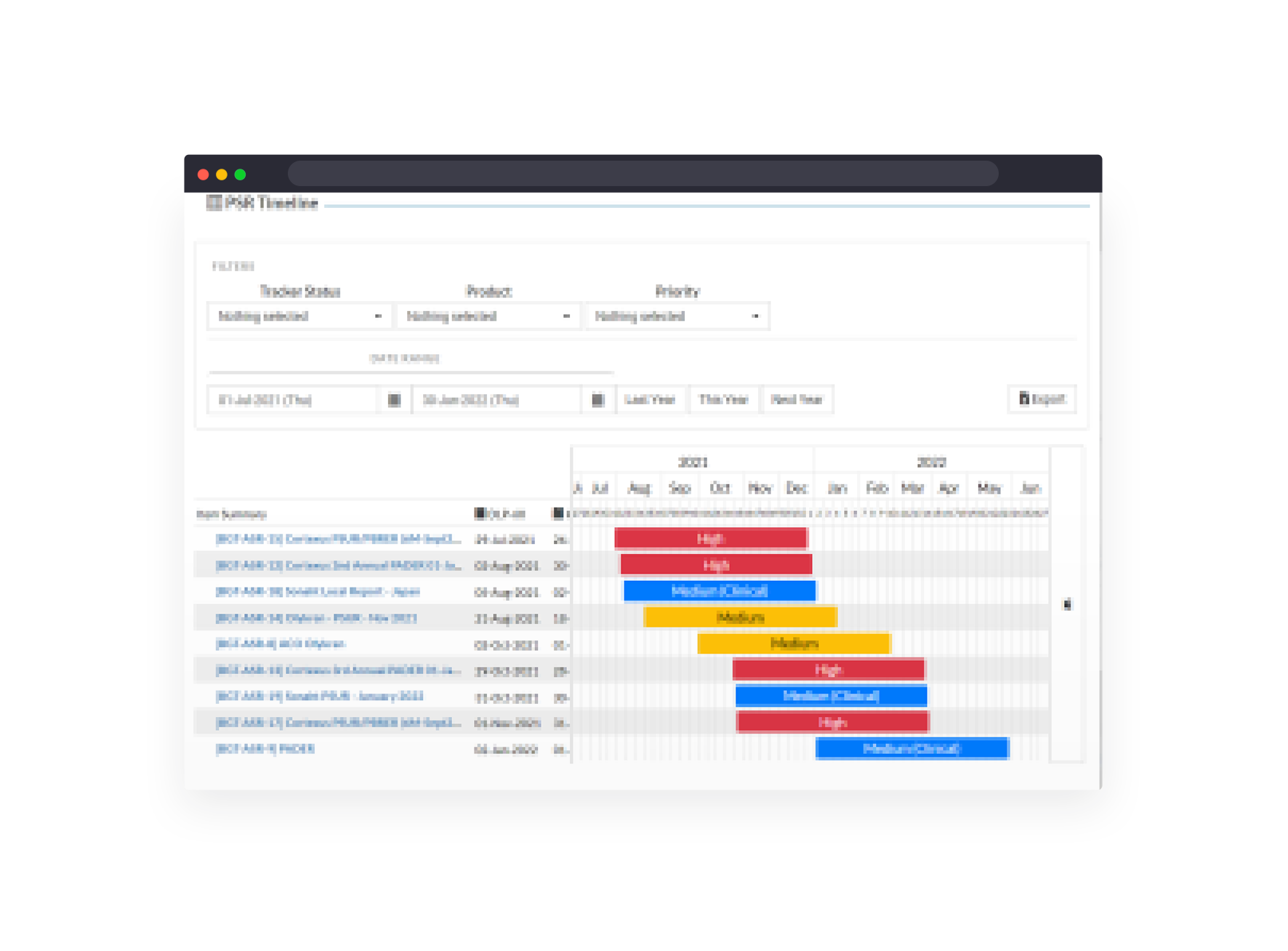
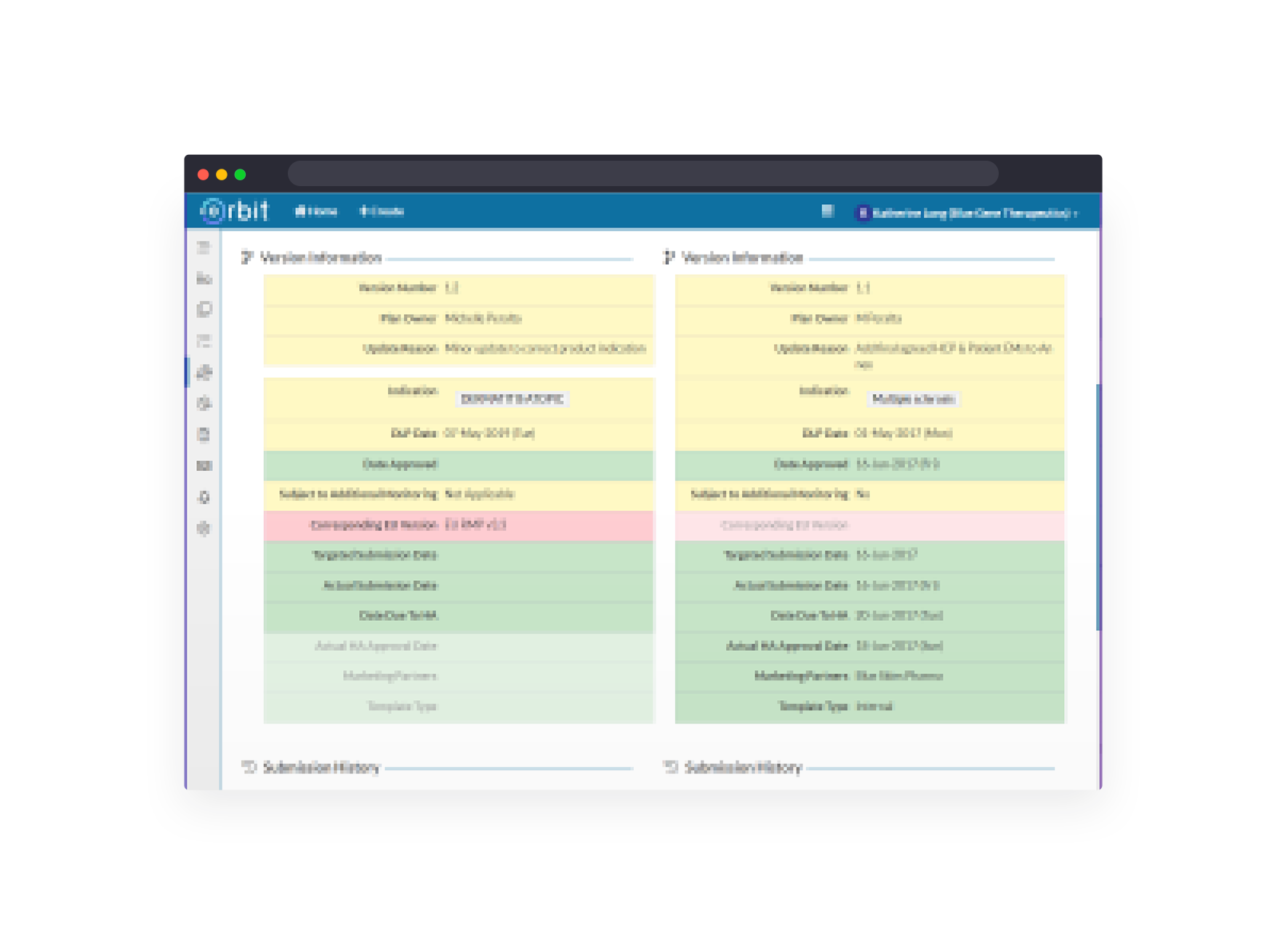
Manage global Aggregate Safety Report schedule, generation, contributions and status across all products.
Orbit’s cross-functional trackers connect PV teams, breaking down data siloes and supporting consistency across the organization.
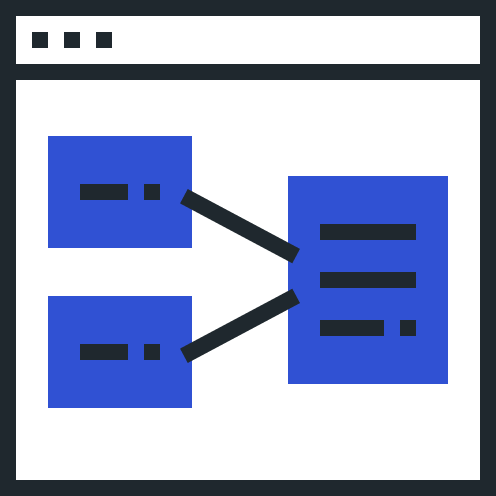
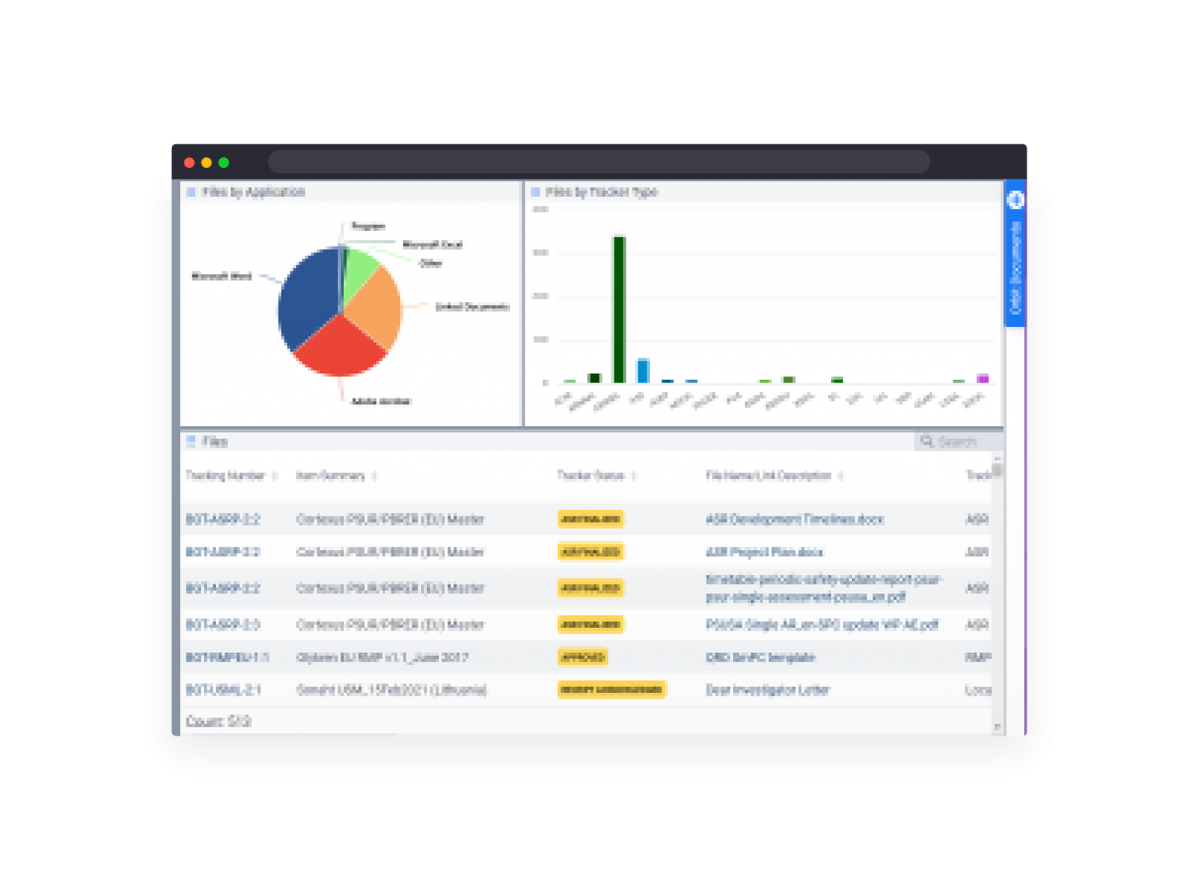
Automate partner document exchange based on your Safety Data Exchange and PV Agreements.
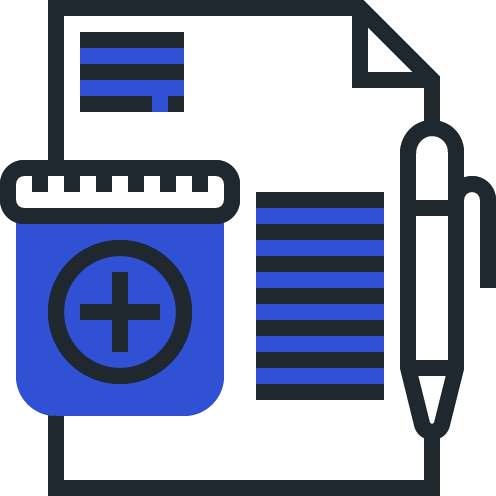
Track Safety Commitments and Health Authority Requests across Regulatory and PV functions.
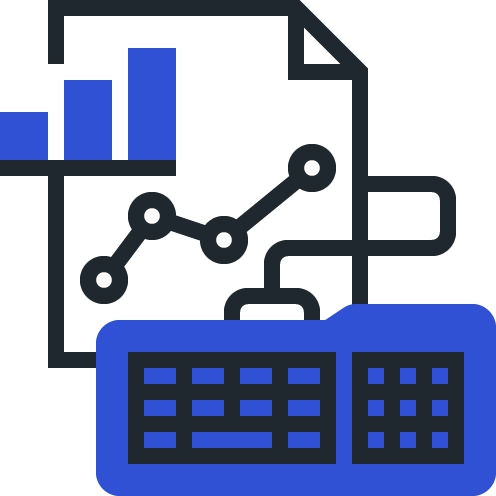
Manage lists of safety concerns and close monitoring events with structured MedDRA query strategies.
Orbit offers the tools your team needs to deliver value across pharmacovigilance, safety, and risk management functional areas.
Guide your users through their work using streamlined and flexible workflow automation.
Simplified reporting on all system data in real-time dashboards and automated snapshot reports.
Orbit is an interconnected system, allowing you to connect related business processes or use them as modular solutions.
Configurable notifications keep your teams aware of their timelines, responsibilities without the manual reminders.
Use AI to gain better insights into your data, documents, and process indicators.
We bring the software, you bring your SOP's: Orbit is fully configurable to your process and requirements.
Browse upcoming and on-demand content from the Orbit webinar series.

WEBINAR Simplify & Harmonize Pharmacovigilance Aggregate Safety Reports Thursday, 15

WEBINAR Additional RMMs, managed: Module XVI Compliance with Orbit February

WEBINAR Global Additional Risk Minimization Measure Implementation: Enable Harmonization, Ensure

WEBINAR Never Miss a Safety-Related Deadline: Automate the Tasking and

WEBINAR Integration and Automation: Leverage Key System Data and Supercharge

WEBINAR Streamlining Local Safety Action Oversight: Automating Global-to-Local Communications, Commitments

New Publication: Tracking a Medicine’s Regulatory Risk Management Commitments Provides Better Transparency

Why QPPVs and global PV leads need better analytics—and how to get

In the complex world of pharmaceutical drug safety, access to the right
Schedule a call today.
Once we’ve received your registration, login information will be sent to your inbox.