
Simplify & Harmonize Pharmacovigilance Aggregate Safety Reports
WEBINAR Simplify & Harmonize Pharmacovigilance Aggregate Safety Reports Thursday, 15
Aggregate Safety Reporting is one of the core functions of Global Patient Safety. Despite the existence of reporting tools, the process remains disjointed and difficult to manage with siloed systems, complex cross-functional handoffs and repetitive manual work.
Orbit’s validated Aggregate Safety Reporting tools takes the guesswork out of Aggregate Reporting, boosting your team’s efficiency with streamlined workflows and automated scheduling.

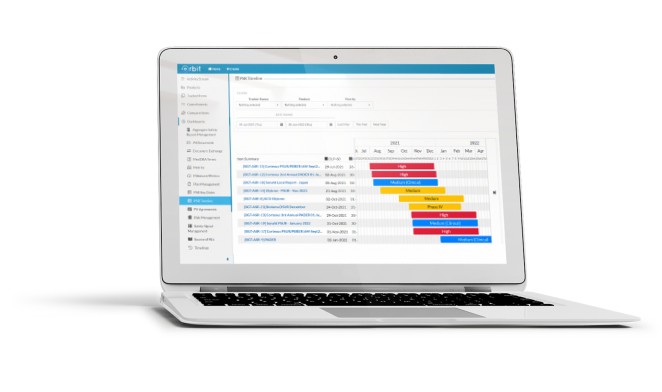
Orbit gives your team the tools needed to schedule and monitor upcoming aggregate safety reports across countries, products, and stakeholders.
Harmonize your global periodic reporting schedule with automated de-duplication across report countries, sources, and products.
Manage reporting projects, calculate milestones and deadlines, and harmonize your global reporting schedule. Orbit’s automated aggregate safety report scheduler makes predicting and monitoring your team’s workload straightforward.
Options to connect with existing RIMS (Regulatory Information Management System) and Document Management to harmonize report scheduling and authoring workflows.
Set the frequency on your Periodic Reports and allow Orbit to schedule upcoming tasks, deadlines, contributions, submissions and other activities around your Data Lock Point.
Orbit captures feedback and follow-up actions from regulators to ensure that you are staying ahead of commitments from Assessment Reports and other health authority requests.
Authoring and review workflows
Document Exchange Automation
Assessment Report Management

WEBINAR Simplify & Harmonize Pharmacovigilance Aggregate Safety Reports Thursday, 15

WEBINAR Holistic Visibility in Aggregate Safety Reporting: Centralize Scheduling for

WEBINAR Aggregate Safety Reporting Optimization: Start Automating At the Source

Pharmacovigilance is a critical part of ensuring patient safety and

The adoption of electronic reporting systems, increased collaboration between stakeholders, and changes in regulatory requirements have all contributed to this shift in proactive safety reporting.

The Challenge The regulatory demands of guidance, PV Legislation, and
Automatically distribute reports to partners based on your Safety Data Exchange and PV Agreements.
Track Safety Commitments and Health Authority Requests related to report submissions and assessments.
With integrated MedDRA browsing, create consistent queries across reporting and data sciences teams.
Schedule a call today.
Once we’ve received your registration, login information will be sent to your inbox.